b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
Application Of O-Chlorophenol:
Chlorophenol, a kind of bactericide and preservative stronger than the phenol, is a kind of important industrial raw material which can also be used as a local disinfectant.
Chlorophenol can be absorbed through skin contact, and it can damage the liver, kidney and lung. It is more poisonous when dissolved in solvent; if inhaled, it can cause accelerated breathing, high blood pressure, fever, increased peristalsis, nervus motorius asthenia, collapse, spasm and even death.
Basic Information Of O-Chlorophenol:
CAS NO: 95-57-8
Molecular weight: 128.5563
Molecular formula: C6H5ClO
Structural formula: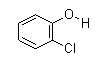
Product Introduction Of O-Chlorophenol:
It is a kind of monochloro derivative of phenol with a formula of ClC6H4OH and with three isomers as o-chlorophenol, m-chlorophenol, and p-chlorophenol. The o-chlorophenol is a kind of colorless liquid with the melting point of 9.0 ° C, the boiling point of 174.9 ° C, the relative density of 1.2634 (20/4 ° C); it is soluble in alcohol, ether, benzene, alkaline aqueous solution, slightly soluble in water. The m-chlorophenol is a kind of colorless solid with the melting point of 33 ° C, the boiling point of 214 ° C, the density of 1.268 g / cm 3 (25 ° C); it is soluble in alcohol, ether, benzene, alkaline aqueous solution. The p-chlorophenol is a kind of colorless solid with the melting point of 43 ~ 44 ° C, boiling point of 219.7 ° C; it is soluble in alcohol, ether, benzene.
The o-and p-chlorophenol are directly derived from the chlorination of phenol. Intra-molecular association reaction occurred in the former, the boiling point of which is lower than the p-chlorophenol in which the intermolecular occurred, so that the two isomers can be separated by distilling. The m-chlorophenol can be produced by other methods. For example: the above-mentioned methods can be utilized in industry and laboratory. When the phenol receives direct chlorination, if the amount of chlorine is large enough, the phenol can generate 2, 4, 6- trichlorophenol. If hypochlorite is used with phenol, it will only generate o-chlorophenol. In carbon tetrachloride, the hypochlorous tert-butyl ester can react with phenol only to generate o-chlorophenol: phenol and sulfuryl chloride SO2Cl2 will react to form o-chlorophenol and p-chlorophenol; after the vacuum distillation, we can get 62% of p-chlorophenol.

