b

Thank You!
Your requirement has been sent, we will contact you quickly!
Sent Failed!
Try again!
Synonymous:14-Dehydroxyl- 11,12-Dehydroandrographolide -3,19- Succinate Potassium Sodium Monohydrate
Structure: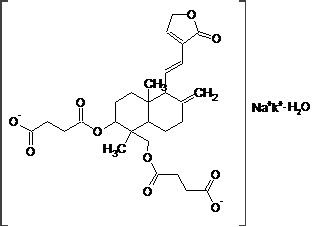
Molecular Formula:C28H34KNaO10·nH2O
Molecular Weight: n=0 592.66 n=1 610.68
NMPA approval No.:H20057578
National Drug Standards:YBH25612005 Draft Quality Standards for Comments(2016)
Test | CP | Results |
Color & Appearance | White to yellowish powder Orcrystalline powder | White powder |
Assay ( HPLC )(calculated by anhydrous substance) | 98%~102% | 99.1% |
Impurities (total) Unkown Impurities(individual) | NMT 2.0 % NMT1.0% | 0.3% 0.08% |
pH | 6.0~7.5 | 6.6 |
Loss on Drying | NMT 4.0 % | 0.9% |
Heavy metals (as Pb) Residual Solvents(acetone ) Bacterial endotoxin | NMT 10 ppm NMT 0.5% LT 0.25EU/mg | Conforms Conforms Conforms |
Package: 1kg
Storage :Preserve in tight, containers ,store at temperature blow 20℃.

